Solubility
Introduction to solubility curves. Discusses compounds dissolved in solutions and factors that affect their ability to do this.
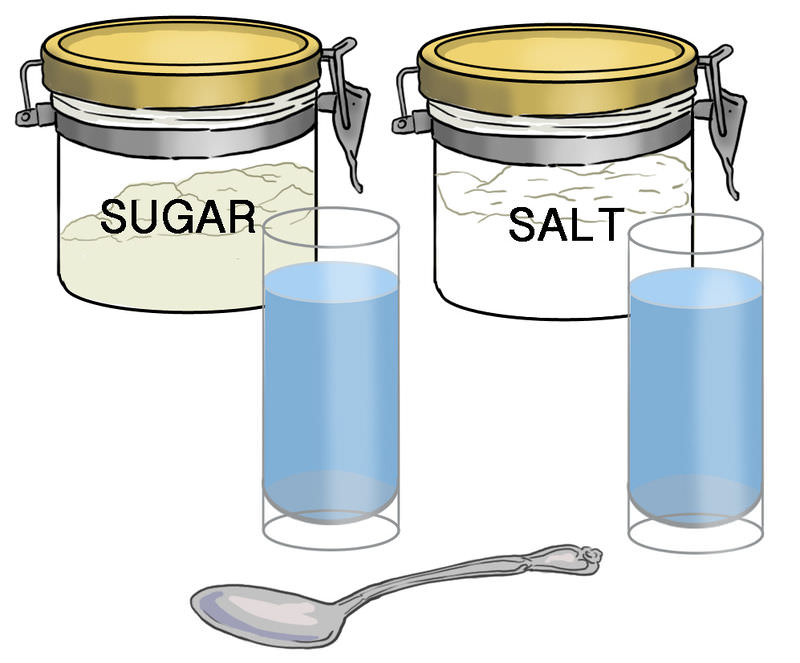
Rhonda wanted to see if salt or sugar dissolves faster in water. She added the same amount of salt and sugar to a half liter of room temperature (20 °C) water in separate glasses. Then she stirred both mixtures. All of the sugar dissolved in less than a minute, but after 5 minutes of stirring, some of the salt still hadn’t dissolved. Even if she had kept stirring the saltwater mixture all day, the remaining salt would not dissolve. Do you know why? The answer is their solubility.
What Is Solubility?
Solubility is the amount of solute that can dissolve in a given amount of solvent at a given temperature. In a solution, the solute is the substance that dissolves, and the solvent is the substance that does the dissolving. For a given solvent, some solutes have greater solubility than others. For example, sugar is much more soluble in water than is salt. But even sugar has an upper limit on how much can dissolve. In a half liter of 20 °C water, the maximum amount is 1000 grams. If you add more sugar than this, the extra sugar won’t dissolve. You can compare the solubility of sugar, salt, and some other solutes in the Table below.
| Solute | Grams of Solute that Will Dissolve in 0.5 L of Water (20 °C) |
|---|---|
| Baking Soda | 48 |
| Epsom salt | 125 |
| Table salt | 180 |
| Table sugar | 1000 |
Q: How much salt do you think Rhonda added to the half-liter of water in her experiment?
A: The solubility of salt is 180 grams per half liter of water at 20 °C. If Rhonda had added less than 180 grams of salt to the half-liter of water, then all of it would have dissolved. Because some of the salt did not dissolve, she must have added more than 180 grams of salt to the water.
Factors That Affect Solubility
Certain factors can change the solubility of a solute. Temperature is one such factor. How temperature affects solubility depends on the state of the solute, as you can see in the Figure below.
- If a solute is a solid or liquid, increasing the temperature increases its solubility. For example, more sugar can dissolve in hot water than in cold water.
- If a solute is a gas, increasing the temperature decreases its solubility. For example, less carbon dioxide can dissolve in warm water than in cold water.
The solubility of gases is also affected by pressure. Pressure is the force pushing against a given area. Increasing the pressure on a gas increases its solubility. Did you ever open a can of soda and notice how it fizzes out of the can? Soda contains dissolved carbon dioxide. Opening the can reduces the pressure on the gas in solution, so it is less soluble. As a result, some of the carbon dioxide comes out of solution and rushes into the air.
Q: Which do you think will fizz more when you open it, a can of warm soda or a can of cold soda?
A: A can of warm soda will fizz more because increasing the temperature decreases the solubility of a gas. Therefore, less carbon dioxide can remain dissolved in warm soda than in cold soda.
Summary
- Solubility is the amount of solute that can dissolve in a given amount of solvent at a given temperature. Some solutes have greater solubility than others.
- Temperature affects the solubility of solutes in all three states. Pressure also affects the solubility of gases.
Review
- What is solubility?
- For a given solute and solvent, what factors affect solubility?
- You open a can of room temperature soda and pour it into a glass. Why does the soda go flat? Will it go flat faster or slower if you add ice? Why?
Explore More
Watch the video about solubility, and then answer the questions below.
- How does Mr. Anderson define solubility in the video?
- What is the solubility of talc in 100 mL of 25 °C water? Explain why talc has this solubility.
- Describe how increasing temperature affects the solubility of solid and liquid solutes.
- Why is there less oxygen in ocean water near the equator than in water at higher latitudes?
- A factory discharges clean, warm water into a nearby stream. Fish keep dying in this part of stream. Explain why.
Resources
Rhonda wanted to see if salt or sugar dissolves faster in water. She added the same amount of salt and sugar to a half liter of room temperature (20 °C) water in separate glasses. Then she stirred both mixtures. All of the sugar dissolved in less than a minute, but after 5 minutes of stirring, some of the salt still hadn’t dissolved. Even if she had kept stirring the saltwater mixture all day, the remaining salt would not dissolve. Do you know why? The answer is their solubility.
What Is Solubility?
Solubility is the amount of solute that can dissolve in a given amount of solvent at a given temperature. In a solution, the solute is the substance that dissolves, and the solvent is the substance that does the dissolving. For a given solvent, some solutes have greater solubility than others. For example, sugar is much more soluble in water than is salt. But even sugar has an upper limit on how much can dissolve. In a half liter of 20 °C water, the maximum amount is 1000 grams. If you add more sugar than this, the extra sugar won’t dissolve. You can compare the solubility of sugar, salt, and some other solutes in the Table below.
| Solute | Grams of Solute that Will Dissolve in 0.5 L of Water (20 °C) |
|---|---|
| Baking Soda | 48 |
| Epsom salt | 125 |
| Table salt | 180 |
| Table sugar | 1000 |
Q: How much salt do you think Rhonda added to the half-liter of water in her experiment?
A: The solubility of salt is 180 grams per half liter of water at 20 °C. If Rhonda had added less than 180 grams of salt to the half-liter of water, then all of it would have dissolved. Because some of the salt did not dissolve, she must have added more than 180 grams of salt to the water.
Factors That Affect Solubility
Certain factors can change the solubility of a solute. Temperature is one such factor. How temperature affects solubility depends on the state of the solute, as you can see in the Figure below.
- If a solute is a solid or liquid, increasing the temperature increases its solubility. For example, more sugar can dissolve in hot water than in cold water.
- If a solute is a gas, increasing the temperature decreases its solubility. For example, less carbon dioxide can dissolve in warm water than in cold water.
The solubility of gases is also affected by pressure. Pressure is the force pushing against a given area. Increasing the pressure on a gas increases its solubility. Did you ever open a can of soda and notice how it fizzes out of the can? Soda contains dissolved carbon dioxide. Opening the can reduces the pressure on the gas in solution, so it is less soluble. As a result, some of the carbon dioxide comes out of solution and rushes into the air.
Q: Which do you think will fizz more when you open it, a can of warm soda or a can of cold soda?
A: A can of warm soda will fizz more because increasing the temperature decreases the solubility of a gas. Therefore, less carbon dioxide can remain dissolved in warm soda than in cold soda.
Summary
- Solubility is the amount of solute that can dissolve in a given amount of solvent at a given temperature. Some solutes have greater solubility than others.
- Temperature affects the solubility of solutes in all three states. Pressure also affects the solubility of gases.
Review
- What is solubility?
- For a given solute and solvent, what factors affect solubility?
- You open a can of room temperature soda and pour it into a glass. Why does the soda go flat? Will it go flat faster or slower if you add ice? Why?
Explore More
Watch the video about solubility, and then answer the questions below.
- How does Mr. Anderson define solubility in the video?
- What is the solubility of talc in 100 mL of 25 °C water? Explain why talc has this solubility.
- Describe how increasing temperature affects the solubility of solid and liquid solutes.
- Why is there less oxygen in ocean water near the equator than in water at higher latitudes?
- A factory discharges clean, warm water into a nearby stream. Fish keep dying in this part of stream. Explain why.












0 Comments